Research Cell
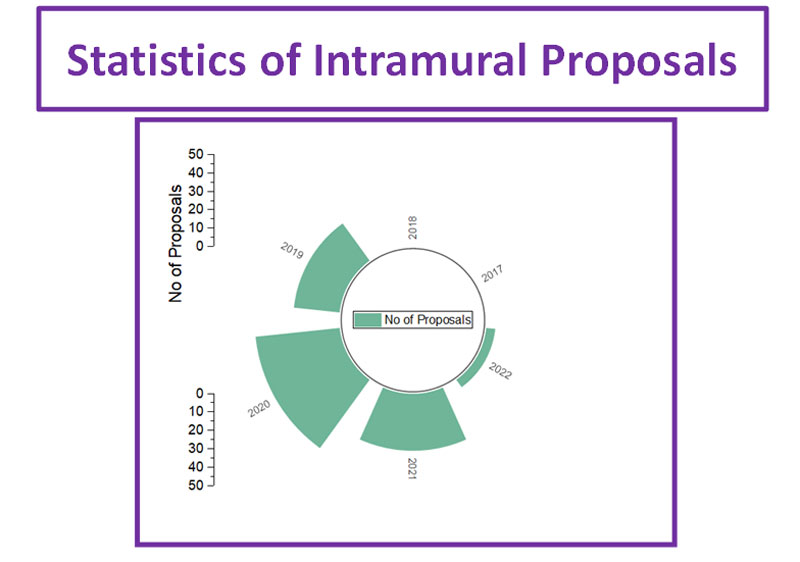
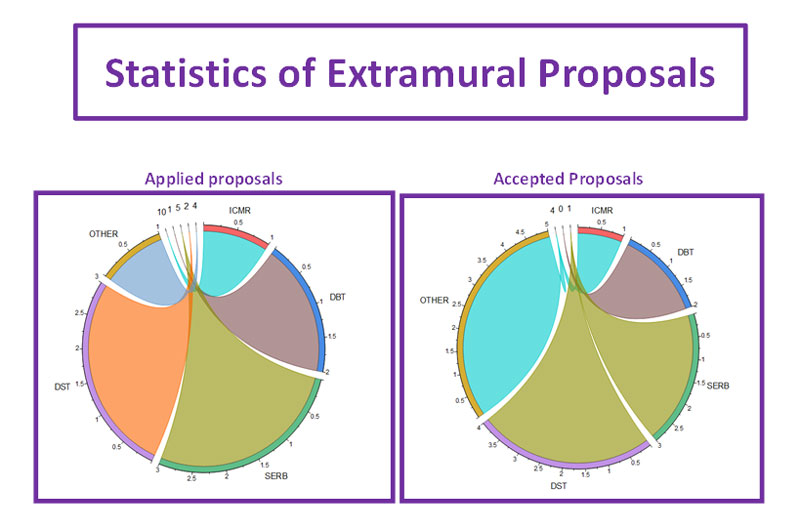
The Department of Research Cell of PGICH, Noida was inaugurated by the Director, Prof. Ajay Singh on 6th April 2022. On this occasion Prof. Ajay explains about the research cell's SOP. He also explained how cell will be beneficial for promoting research at PGICH. He announced that the PGICH faculty and students of the institute have been trained by the research cell to make them proficient in effective writing of research papers/proposals, bio-statistical analysis etc to get financial assistance from various government agencies like DBT, ICMR, SIRB, DST, UPCST, WHO etc. Recently, the research cell has also call for intramural funds, which will be examined by an external committee.
On this occasion, the Dean of the Institute, Prof. Jyotsna Madan, CMS Prof. DK Singh, MS Dr. Akash Raj, in-charge Research Cell, Prof. Ruchi Rai, Deputy In-charge Research Cell, Dr. Dinesh Kumar Sahu, member Dr. Mayank Nilay and other faculty members were present.
1. Responsibility
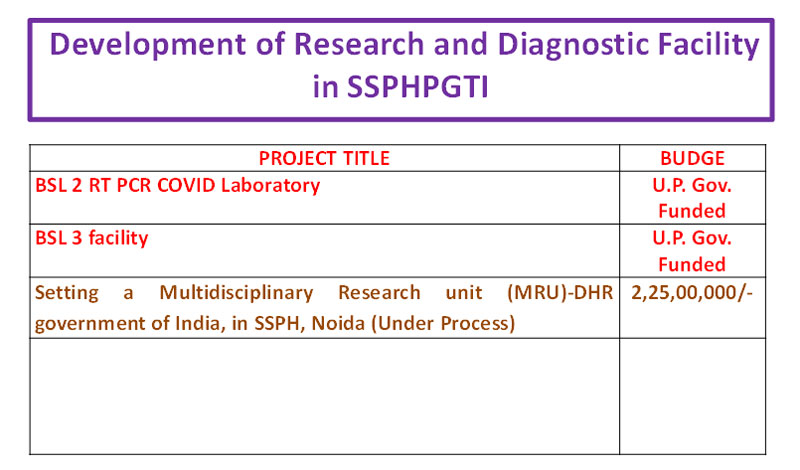
Medical research has become increasingly important given the ever‐growing burden of diseases and the urgent need to find new and cost effective ways to improve the health of the people. One of the major objectives of Post Graduate Institute of Child Health, NOIDA is Research. It is the responsibility of Faculty, Residents and Technical Staff to engage in research in clinical, laboratory and basic sciences.
2. Research and Ethics
It is of paramount importance to ensure that research is conducted in the best interests of the patients and participants and in a fair, honest, and transparent manner. The major ethical principles that apply to biomedical research are: Autonomy of the patient/participant, Benefit of the patient/participant, Justice and avoid maleficence. Ethical clearances for all Intramural and Extramural Research Projects shall be dealt with by the Institutional Ethical Committee (IEC), PGICH. The responsibilities of the Research Cell are to ensure that following by Research committee/Ethics Committee:
- The research study protocols are sound in design, scientifically justified, and statistically valid.
- The research studies are conducted according to the Indian Council of Medical Research and Good Clinical and Laboratory Practice guidelines.
- The research study does not compromise rights, safety and benefits of the patients/ volunteers/ study participants or violates animal rights.
- The research studies are conducted under the supervision of trained medical/bio‐medical persons with required expertise.
- The research studies include only those patients or participants who have given voluntary and informed consent without any inducement or coercion.
- Ethical approval of a research study: Each research proposal must be submitted to the IEC for approval.
- It should be ensured that no research project is started unless Ethics Clearance / approval is obtained and that no retrospective/Post facto Ethics Clearance/Approval can be provided to research projects which were neither submitted nor vetted by the Institute Ethics Committee.






Research Committee
| # |
Title |
Details |
|
The revised constitution of Research Committee, Oct 16, 2019 |
 |
Institutional Ethical Committee
| # |
Title |
Details |
|
The revised constitution of Ethical Committee, Oct 16, 2019 |
 |
|
Guideline for Presentation on IEC/IRC, SSPHPGTI |
 |
|
SOP of Institutional Ethics Committee of SSPHPGTI, July 2019 |
 |
|
Application form IEC |
 |
|
Consent Waiver Form |
 |
|
Undertaking by PI |
 |
SOP of IEC
| # |
Title |
Details |
|
SOP of IEC |
 |
